Sirolimus-eluting coronary stent system iVascular angiolite
Sirolimus-eluting coronary stent system iVascular angiolite
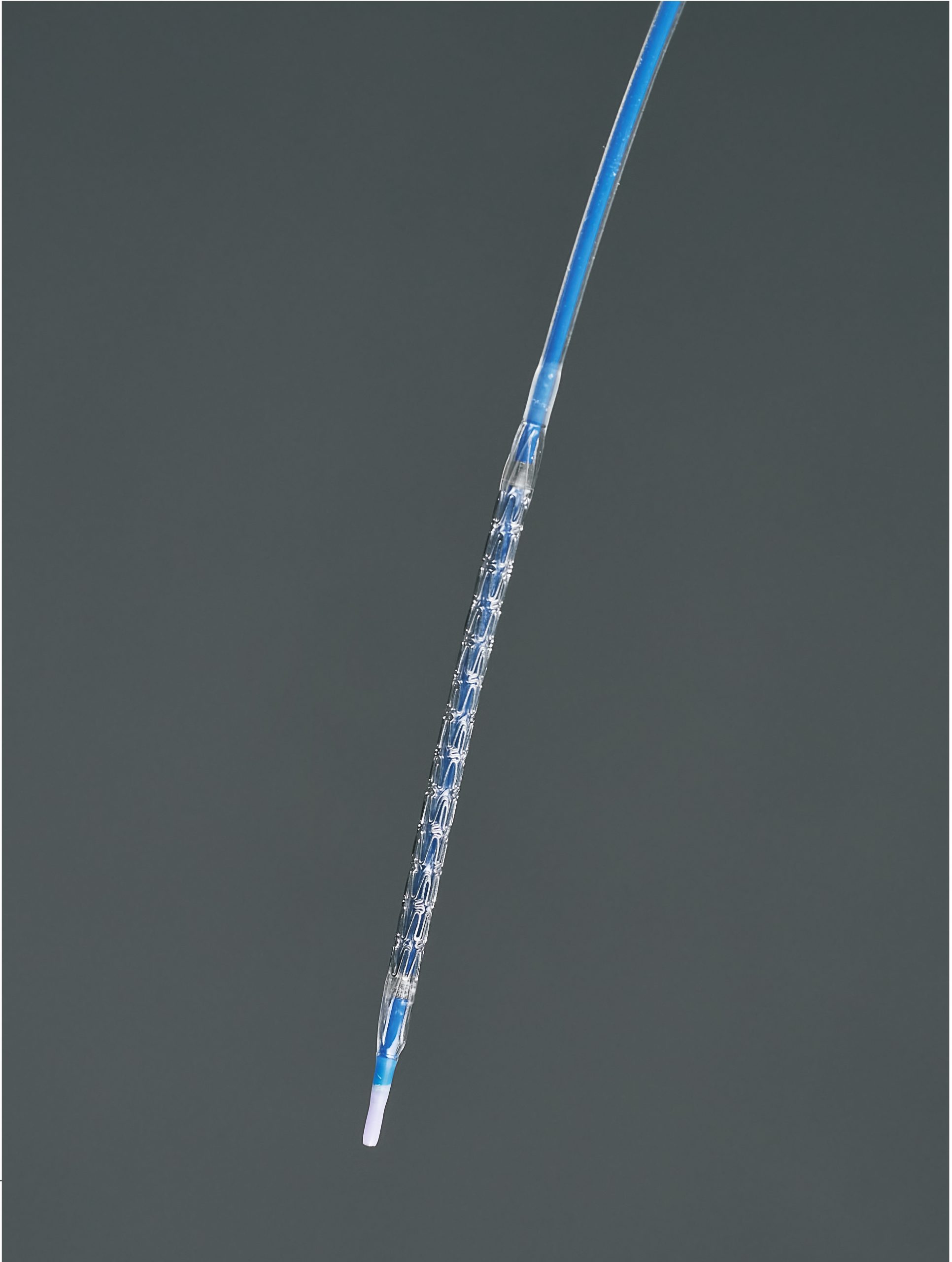
The iVascular angiolite sirolimus-eluting coronary stent is a stent made of cobalt-chromium alloy L605 coated with sirolimus and polymers. The stent is supplied pre-assembled in a system that expands it in the coronary artery to be manipulated via a distally positioned balloon. The stent is designed to fit arteries of various diameters thanks to its adaptable open-cell design with alternating connectors. The stent is manufactured by laser cutting metal tubes. The larger stents (4.00×49 mm) have a stent drug load of approximately 1.4 μg/mm2, corresponding to a maximum drug load of 433 μg. The stent delivery system is a rapid-change balloon catheter. There are marks on the catheter body that allow for correct calculation of the catheter positioning when it is advanced through the guiding catheter without fluoroscopy so that when the last mark disappears, the catheter will be near the tip of the guiding catheter and near the entrance to the artery. The mark located near the catheter connector is intended for femoral access guiding catheters, and the remote mark is for brachial access guiding catheters. The distal part of the catheter has a wear-resistant hydrophilic coating, which provides the catheter with lubrication for passage through the arteries.
The working length of the catheter is 142 cm, the total length is 150 cm. The stent diameter is from 2.00 to 4.50 mm, the length is from 9 to 49 mm.
Indications for use
This device is intended to increase the internal diameter of an artery to improve blood flow in the following cases:
For manipulation of arteries in patients with symptomatic coronary artery disease caused by de novo stenosis or restenosis. Arterial diameter 2-4.5 mm.
For manipulation of arteries in patients with occlusion due to acute myocardial infarction. Arterial diameter 2-4.5 mm.
Дополнительные сведения
| Производитель | |
|---|---|
| Страна Производитель | Испания |
The iVascular angiolite sirolimus-eluting coronary stent is a stent made of cobalt-chromium alloy L605 coated with sirolimus and polymers. The stent is supplied pre-assembled in a system that expands it in the coronary artery to be manipulated via a distally positioned balloon. The stent is designed to fit arteries of various diameters thanks to its adaptable open-cell design with alternating connectors. The stent is manufactured by laser cutting metal tubes. The larger stents (4.00×49 mm) have a stent drug load of approximately 1.4 μg/mm2, corresponding to a maximum drug load of 433 μg. The stent delivery system is a rapid-change balloon catheter. There are marks on the catheter body that allow for correct calculation of the catheter positioning when it is advanced through the guiding catheter without fluoroscopy so that when the last mark disappears, the catheter will be near the tip of the guiding catheter and near the entrance to the artery. The mark located near the catheter connector is intended for femoral access guiding catheters, and the remote mark is for brachial access guiding catheters. The distal part of the catheter has a wear-resistant hydrophilic coating, which provides the catheter with lubrication for passage through the arteries.
The working length of the catheter is 142 cm, the total length is 150 cm. The stent diameter is from 2.00 to 4.50 mm, the length is from 9 to 49 mm.
Indications for use
This device is intended to increase the internal diameter of an artery to improve blood flow in the following cases:
For manipulation of arteries in patients with symptomatic coronary artery disease caused by de novo stenosis or restenosis. Arterial diameter 2-4.5 mm.
For manipulation of arteries in patients with occlusion due to acute myocardial infarction. Arterial diameter 2-4.5 mm.
The iVascular angiolite sirolimus-eluting coronary stent is a stent made of cobalt-chromium alloy L605 coated with sirolimus and polymers. The stent is supplied pre-assembled in a system that expands it in the coronary artery to be manipulated via a distally positioned balloon. The stent is designed to fit arteries of various diameters thanks to its adaptable open-cell design with alternating connectors. The stent is manufactured by laser cutting metal tubes. The larger stents (4.00×49 mm) have a stent drug load of approximately 1.4 μg/mm2, corresponding to a maximum drug load of 433 μg. The stent delivery system is a rapid-change balloon catheter. There are marks on the catheter body that allow for correct calculation of the catheter positioning when it is advanced through the guiding catheter without fluoroscopy so that when the last mark disappears, the catheter will be near the tip of the guiding catheter and near the entrance to the artery. The mark located near the catheter connector is intended for femoral access guiding catheters, and the remote mark is for brachial access guiding catheters. The distal part of the catheter has a wear-resistant hydrophilic coating, which provides the catheter with lubrication for passage through the arteries.
The working length of the catheter is 142 cm, the total length is 150 cm. The stent diameter is from 2.00 to 4.50 mm, the length is from 9 to 49 mm.
Indications for use
This device is intended to increase the internal diameter of an artery to improve blood flow in the following cases:
For manipulation of arteries in patients with symptomatic coronary artery disease caused by de novo stenosis or restenosis. Arterial diameter 2-4.5 mm.
For manipulation of arteries in patients with occlusion due to acute myocardial infarction. Arterial diameter 2-4.5 mm.
No technical specifications available.