Sirolimus-eluting coronary stent system M’Sure-S
Sirolimus-eluting coronary stent system M’Sure-S
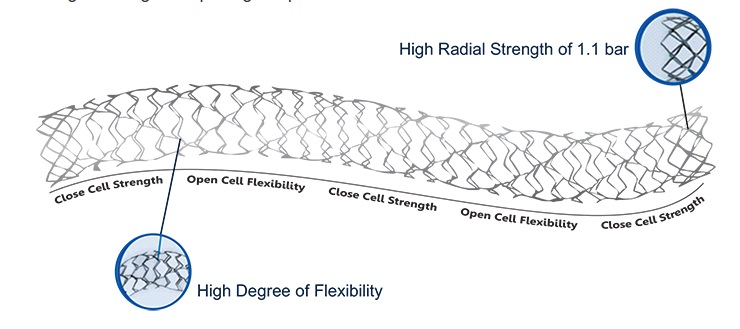
The M’Sure-S sirolimus-eluting coronary stent system is a combination product consisting of two adjustable components: the device (coronary stent system) and the drug (sirolimus contained in a polymer coating) pre-positioned on a balloon catheter between two platinum radiopaque marker bands.
Stent material: Surgical grade cobalt chrome (L605), laser cut from a seamless serpentine/spiral tube coated with a mixture of polymer and sirolimus.
Stent diameter from 2 mm to 5 mm, stent length from 8 mm to 48 mm.
Usable length of the delivery system: 140 cm
Delivery system Y-adapter ports: One access port to the lumen of the
inflation/deflation. The exit port for the guidewire is located at a distance of 25 cm from the tip. Designed for a 0.014” (0.36 mm) guidewire.
Stent Delivery Balloon: Polyamide balloon, nominally 1.0 mm longer than the stent, the length and location of the deployed stent is determined by a radiopaque marker proximal and distal to the stent.
The active pharmaceutical ingredient in the M’Sure-S sirolimus-eluting coronary stent is sirolimus. Sirolimus is a macrolide antibiotic derived from a strain of bacteria (streptomyces hygroscopicus). Its molecular formula is C51H79NO13, molecular weight is 914.19 g/mol.
M’Sure-S is indicated for improving coronary vessel lumen diameter in patients with symptomatic ischemic heart disease with de novo coronary lesions in native coronary arteries with a reference vessel diameter of 2.50 mm to 4.0 mm and a lesion length d” 36 mm. M’Sure-S is intended for use in patients with indications for percutaneous transluminal coronary angioplasty (PTCA).
Дополнительные сведения
| Производитель | |
|---|---|
| Страна Производитель | Индия |
The M’Sure-S sirolimus-eluting coronary stent system is a combination product consisting of two adjustable components: the device (coronary stent system) and the drug (sirolimus contained in a polymer coating) pre-positioned on a balloon catheter between two platinum radiopaque marker bands.
Stent material: Surgical grade cobalt chrome (L605), laser cut from a seamless serpentine/spiral tube coated with a mixture of polymer and sirolimus.
Stent diameter from 2 mm to 5 mm, stent length from 8 mm to 48 mm.
Usable length of the delivery system: 140 cm
Delivery system Y-adapter ports: One access port to the lumen of the
inflation/deflation. The exit port for the guidewire is located at a distance of 25 cm from the tip. Designed for a 0.014” (0.36 mm) guidewire.
Stent Delivery Balloon: Polyamide balloon, nominally 1.0 mm longer than the stent, the length and location of the deployed stent is determined by a radiopaque marker proximal and distal to the stent.
The active pharmaceutical ingredient in the M’Sure-S sirolimus-eluting coronary stent is sirolimus. Sirolimus is a macrolide antibiotic derived from a strain of bacteria (streptomyces hygroscopicus). Its molecular formula is C51H79NO13, molecular weight is 914.19 g/mol.
M’Sure-S is indicated for improving coronary vessel lumen diameter in patients with symptomatic ischemic heart disease with de novo coronary lesions in native coronary arteries with a reference vessel diameter of 2.50 mm to 4.0 mm and a lesion length d” 36 mm. M’Sure-S is intended for use in patients with indications for percutaneous transluminal coronary angioplasty (PTCA).
The M’Sure-S sirolimus-eluting coronary stent system is a combination product consisting of two adjustable components: the device (coronary stent system) and the drug (sirolimus contained in a polymer coating) pre-positioned on a balloon catheter between two platinum radiopaque marker bands.
Stent material: Surgical grade cobalt chrome (L605), laser cut from a seamless serpentine/spiral tube coated with a mixture of polymer and sirolimus.
Stent diameter from 2 mm to 5 mm, stent length from 8 mm to 48 mm.
Usable length of the delivery system: 140 cm
Delivery system Y-adapter ports: One access port to the lumen of the
inflation/deflation. The exit port for the guidewire is located at a distance of 25 cm from the tip. Designed for a 0.014” (0.36 mm) guidewire.
Stent Delivery Balloon: Polyamide balloon, nominally 1.0 mm longer than the stent, the length and location of the deployed stent is determined by a radiopaque marker proximal and distal to the stent.
The active pharmaceutical ingredient in the M’Sure-S sirolimus-eluting coronary stent is sirolimus. Sirolimus is a macrolide antibiotic derived from a strain of bacteria (streptomyces hygroscopicus). Its molecular formula is C51H79NO13, molecular weight is 914.19 g/mol.
M’Sure-S is indicated for improving coronary vessel lumen diameter in patients with symptomatic ischemic heart disease with de novo coronary lesions in native coronary arteries with a reference vessel diameter of 2.50 mm to 4.0 mm and a lesion length d” 36 mm. M’Sure-S is intended for use in patients with indications for percutaneous transluminal coronary angioplasty (PTCA).
No technical specifications available.